
The biopharmaceutical manufacturing landscape has undergone significant changes over the past decade due to the rapid adoption of polymer-based single-use technologies. Traditionally, pharmaceutical companies relied on stainless steel equipment for the development and production of biotherapeutics. However, stainless steel is not only expensive and inflexible, but it also necessitates time-consuming cleaning-in-place (CIP) and sterilization-in-place (SIP) procedures, which can inadvertently increase the risk of contamination. Single-use solutions, including bags, tubing, connectors, and tube sets, are transforming the biopharmaceutical manufacturing landscape. As pre-sterilized and highly flexible technologies, single-use solutions provide manufacturers with simple, customizable, and cost-effective options to address the production challenges associated with a diverse range of drug products. In contrast to traditional, rigid, costly, and labor-intensive production lines based on stainless steel systems, single-use solutions are designed to streamline production processes and accelerate time to market.
The benefits of single-use technology are numerous and include:
Ensuring Sterility: Biopharmaceutical manufacturers that utilize stainless steel equipment in their processes must clean and sterilize it after each use. Traditionally, this is accomplished through steam cleaning, a method that is both time-consuming and energy-intensive. In contrast, single-use technology products are pre-sterilized, significantly reducing the initial risk of microbial contamination. Consequently, these single-use products enable manufacturers to uphold high standards of product quality, enhance patient safety, and streamline their sterilization validation processes.
Enhancing Process Flexibility: Stainless steel systems typically necessitate dedicated production lines, often designed for a single product or production step. For biopharmaceutical companies, this results in reduced flexibility and demands significant time investment to implement complex modifications when reconfiguring the process flow. In contrast, the diverse specifications of single-use components allow for swift and straightforward adjustments, facilitating rapid process changes. This adaptability enables manufacturers to maintain agility and respond promptly to evolving process requirements.
Enhancing Operational Efficiency: In addition to eliminating the need for cleaning and sterilization, single-use technology simplifies equipment construction and setup. Typically, single-use systems can seamlessly integrate bags, tubes, connectors, filters, and other components into upstream and downstream processes. This integration allows operators to concentrate on high-value tasks, thereby maximizing productivity and output. Improving operational efficiency is a crucial driver in the biopharmaceutical industry, enabling developers to meet the increasing demand for biopharmaceutical production.
Avoid Cross-Contamination: In traditional stainless steel systems, cross-contamination poses a significant risk, as it can compromise both the quality and integrity of the production process and the final product. Single-use technology is designed for one-time use, effectively eliminating the risk of contamination residues during production. Once a single-use component has been utilized, it can be discarded and replaced with a new sterile product.
Reducing Production Costs: Single-use technology eliminates the need for extensive cleaning and validation procedures, allowing biopharmaceutical manufacturers to save on costs and labor while achieving an early return on investment. For instance, single-use cell culture bags are significantly more cost-effective than the maintenance, cleaning, and sterilization resources required for traditional stainless steel bioreactors. A consistent and reliable supply chain for single-use solutions optimizes production schedules, minimizes downtime, and enhances overall process efficiency, thereby accelerating the time to market for drugs.
Compliance: In the biopharmaceutical industry, adherence to regulatory and clinical standards is essential. Pre-sterilized single-use technology is traceable, as it is accompanied by comprehensive quality and regulatory documentation, which can streamline compliance efforts and mitigate risks. Opting for single-use products and collaborating with reputable suppliers can reduce the likelihood of undesirable chemical components and decrease particulate matter, thereby addressing potential safety and efficacy concerns in the final product.
Reduced Environmental Impact: Traditional pharmaceutical production facilities that utilize stainless steel require substantial energy and employ various chemicals for equipment cleaning and sterilization. This not only impacts the environment but also incurs high disposal costs. In contrast, single-use materials can significantly decrease water consumption and minimize the use of corrosive and acidic chemicals in Clean-in-Place (CIP) and Sterilize-in-Place (SIP) procedures.
Once the decision to adopt single-use technology has been made, the next consideration is how to select the single-use technology. Single-use products encompass a variety of polymer materials, each designed for specific purposes based on the type of device and its role in the production process. The materials utilized in single-use technology must endure all bioprocessing conditions, including extreme temperatures, pH fluctuations, pressure variations, changes in flow rates, functional performance, sterilization, and the requirements for storage and transportation. Understanding the physical properties of the chosen materials will enable manufacturers to develop the most effective therapies. For instance, certain polymers may offer the desired biological inertness and temperature tolerance for single-use bags but may lack the durability needed for long-distance transportation. Additionally, to prevent punctures, material manufacturers may apply a protective layer of plastic film on the exterior using various polymers. These material considerations are crucial when developing effective single-use technology products for specific applications, including:
Chemical Composition, Extractables, and Leachables: Single-use technology products must be constructed from bioinert materials, meaning they are biocompatible, to prevent adverse reactions with process fluids that come into contact with them, ultimately safeguarding the patient. The chemical composition of these materials is also critical. Biopharmaceutical manufacturers should prioritize materials with low extractables to minimize the risk of leachables, which can significantly impact the final product. Certain additives in single-use materials may leach into the fluids during formulation, transfer, and storage, potentially compromising the safety of the drug product.
Temperature Tolerance: Most polymer materials are unsuitable for use in extremely cold or hot conditions. However, certain types of films can be engineered to withstand specific temperature ranges, such as -80°C for cryogenic storage or -196°C for liquid nitrogen freezing.
Durability: The materials used in single-use products must be sufficiently durable to prevent excessive damage, scratches, or punctures during daily care and handling. In addition to durability, it is essential to maintain high quality and consistency for each product, as any defects may adversely affect the results of the process.
Clinical Safety and Regulatory Compliance: When developing a bioprocess, one critical aspect of process design that is often overlooked is clinical safety. This consideration is particularly important when evaluating single-use solutions. The materials used to manufacture these components are frequently subject to stringent guidelines and regulations. A thorough understanding of the relevant regulations can assist biopharmaceutical manufacturers in selecting the most optimized single-use solutions to safely conduct clinical trials.
Film Characteristics: The choice between single-layer and multi-layer films largely depends on the specific single-use technology product and its intended application. Multi-layer films can create a gas barrier, enhancing durability and improving material compatibility. Additionally, optical clarity is essential in single-use processes that require visual indication or measurement. Films designed for these purposes typically offer the necessary transparency and high clarity.
The most common and widely used polymers in contemporary single-use technology products are designed to endure a range of conditions, including extreme temperature variations, mechanical flexibility, and exposure to harsh chemicals. These polymers are utilized in a diverse array of products, from filters and bags to tubing and accessories. Furthermore, engineers specializing in single-use technology development can fine-tune these polymer materials to meet specific application requirements.
In response to the needs of different process applications in biopharmaceutical manufacturing, Duoning has independently developed three film products: Duofilm?-001, Duofilm?-002 and Duofilm?-003 to adapt to the characteristics of different process conditions.
The DuoFilm?-001 film independently developed by Duoning is co-extruded 7 layers film of nylon (PA), low-density polyethylene (LDPE), ethylene-vinyl alcohol copolymer (EVOH) and ultra-low-density polyethylene (ULDPE). It has a thickness of 0.3 mm. The outer PA layer provides strong puncture resistance, strength and stability; the EVOH layer minimizes the diffusion of gas on the film while maintaining very good flex crack resistance; the liquid contact layer is ULDPE, which has excellent physical and chemical properties and biocompatibility.
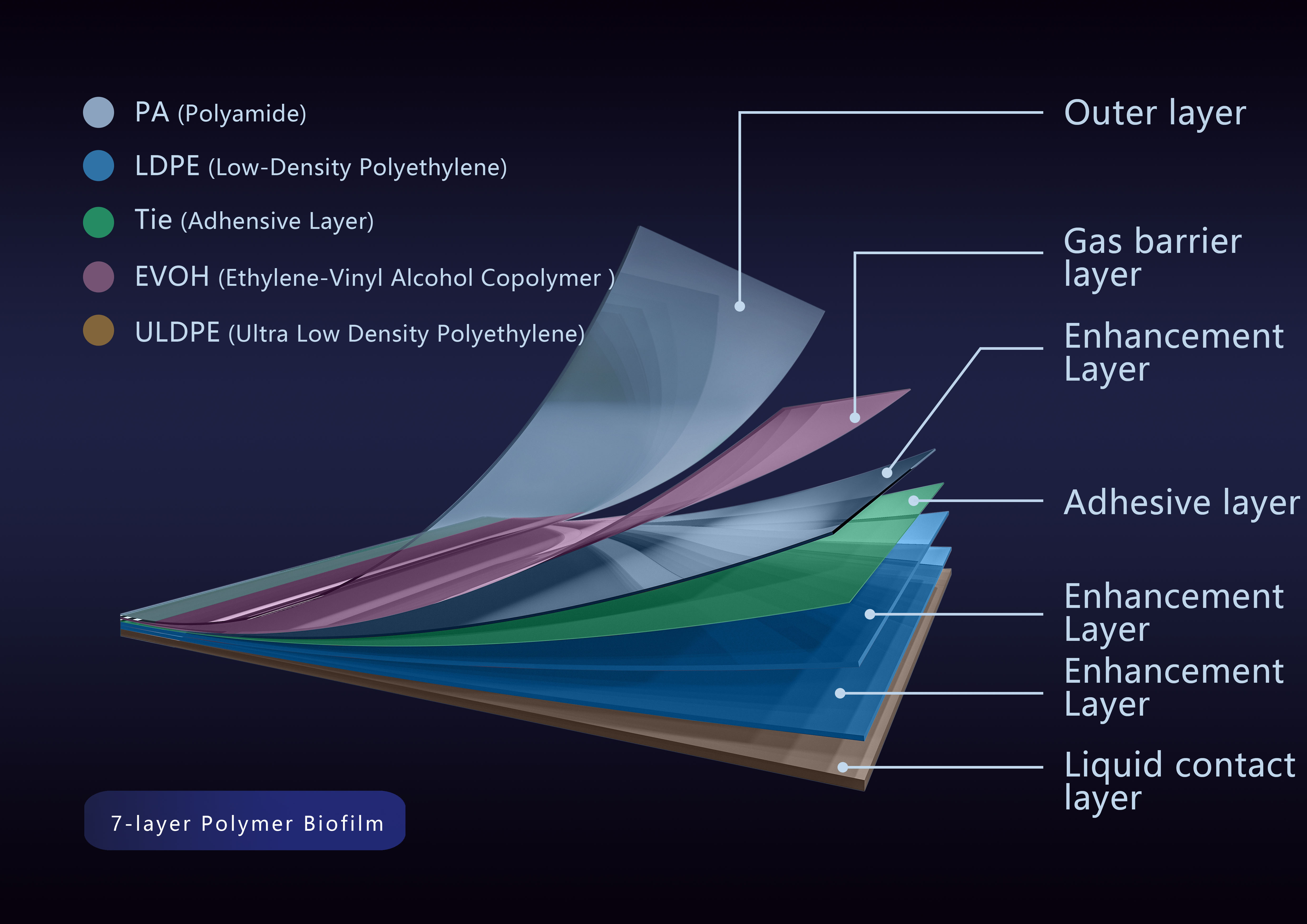
DuoFilm?-002 multi-layer structure diagram
DuoFilm?-001 is mainly used for 2D liquid storage bags, 3D drums or 3D cubic liquid storage bags, open liner bags, feeding bags, mixing bags. These products are produced and packaged in ISO 14644-1:2015 Class 7 cleanrooms, comply with relevant standards including USP, FDA, ISO and European Pharmacopoeia, and are tested for extractables by internationally renowned third-party testing laboratories in accordance with BPOG standards. The products can ensure that the raw materials, product assembly, sterilization and other manufacturing steps are fully in compliance with cGMP and biopharmaceutical operation requirements.
DuoFilm?-002 is a 7-layer co-extruded film composed of polyester elastomer, ethylene-vinyl alcohol copolymer and ultra-low density polyethylene, with a thickness of 0.325 mm and a liquid contact layer of ULDPE. It has high strength, high toughness, low-temperature resistance and aging resistance, and can be used for frozen storage at -80°C.
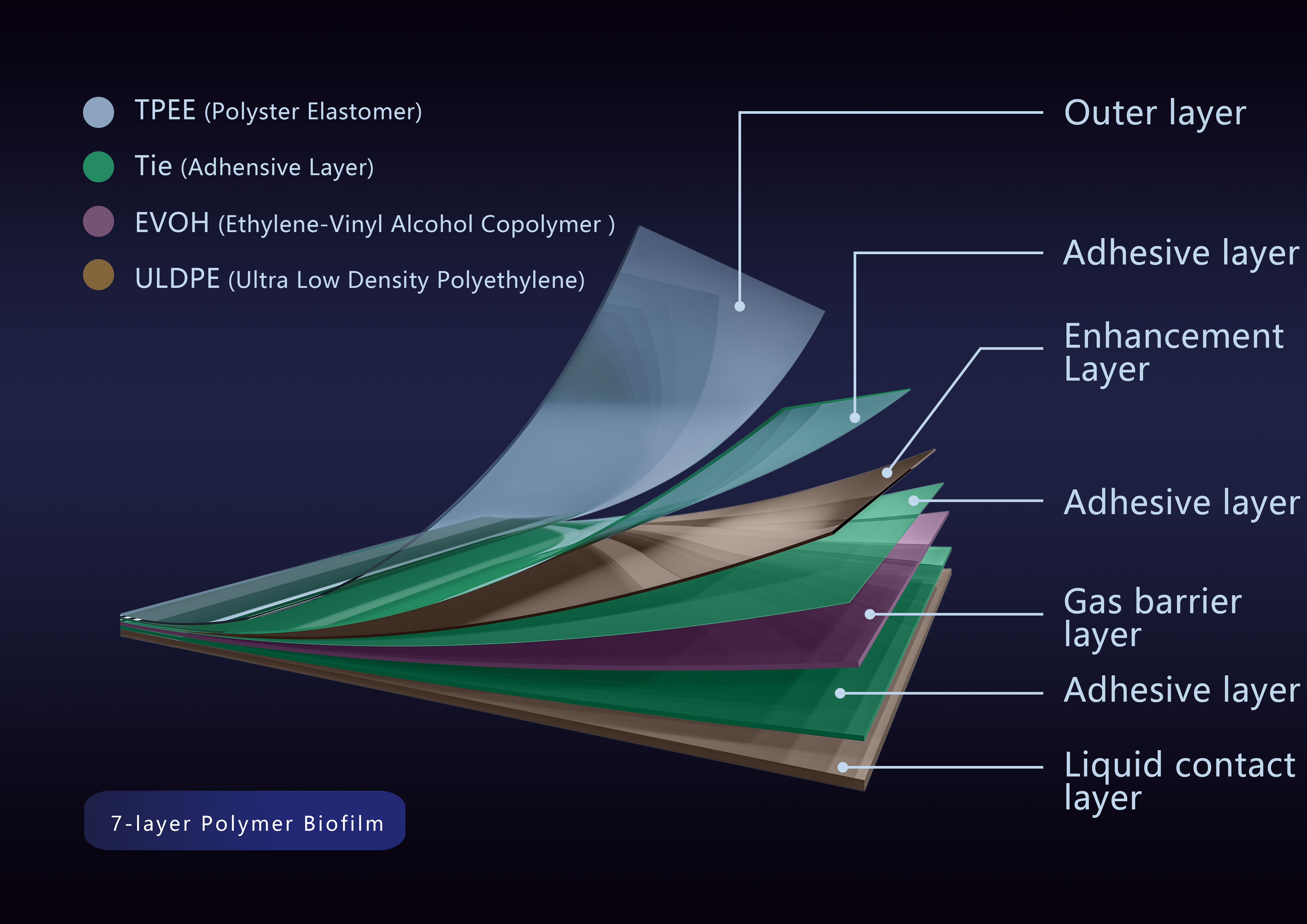
DuoFilm?-002 multi-layer structure diagram
DuoFilm?-002 is mainly used for GlacierStore? single-use freeze-thaw bags independently developed by Duoning. This series of products is produced and packaged in cleanroom that complies with ISO 14644-1:2015 Class 7, and complies with relevant standards including USP, FDA, ISO and European Pharmacopoeia. It is tested for extractables by an internationally renowned third-party testing laboratory in accordance with BPOG standards. After freezing tests, in which the product was irradiated, frozen at -80℃ and thawed at room temperature, it was found that all performance data was not affected, which met the relevant standards. GlacierStore? single-use freeze-thaw bags adopt a “bag + shell” pre-assembled structure, which can be designed as a manifold with multiple bags and used with automated bulk filling system to achieve high-precision and automated filling of bulk drug substance.
DuoFilm?-003 is co-extruded from 5 layers of polymers including low-density polyethylene, ethylene-vinyl alcohol copolymer and ultra-low-density polyethylene. The outer layer of low-density polyethylene (LDPE) has excellent chemical stability and elongation; the middle layer of ethylene-vinyl alcohol copolymer EVOH has excellent barrier effects on gases, odors, and solvents, while maintaining flexibility and strength performance; the liquid contact layer ultra-low-density polyethylene (ULDPE) has excellent biocompatibility and low extractable levels, a clean, inert surface, and no cell growth inhibition additive. This series of membranes supports the most stringent physical requirements while showing excellent cell culture compatibility.
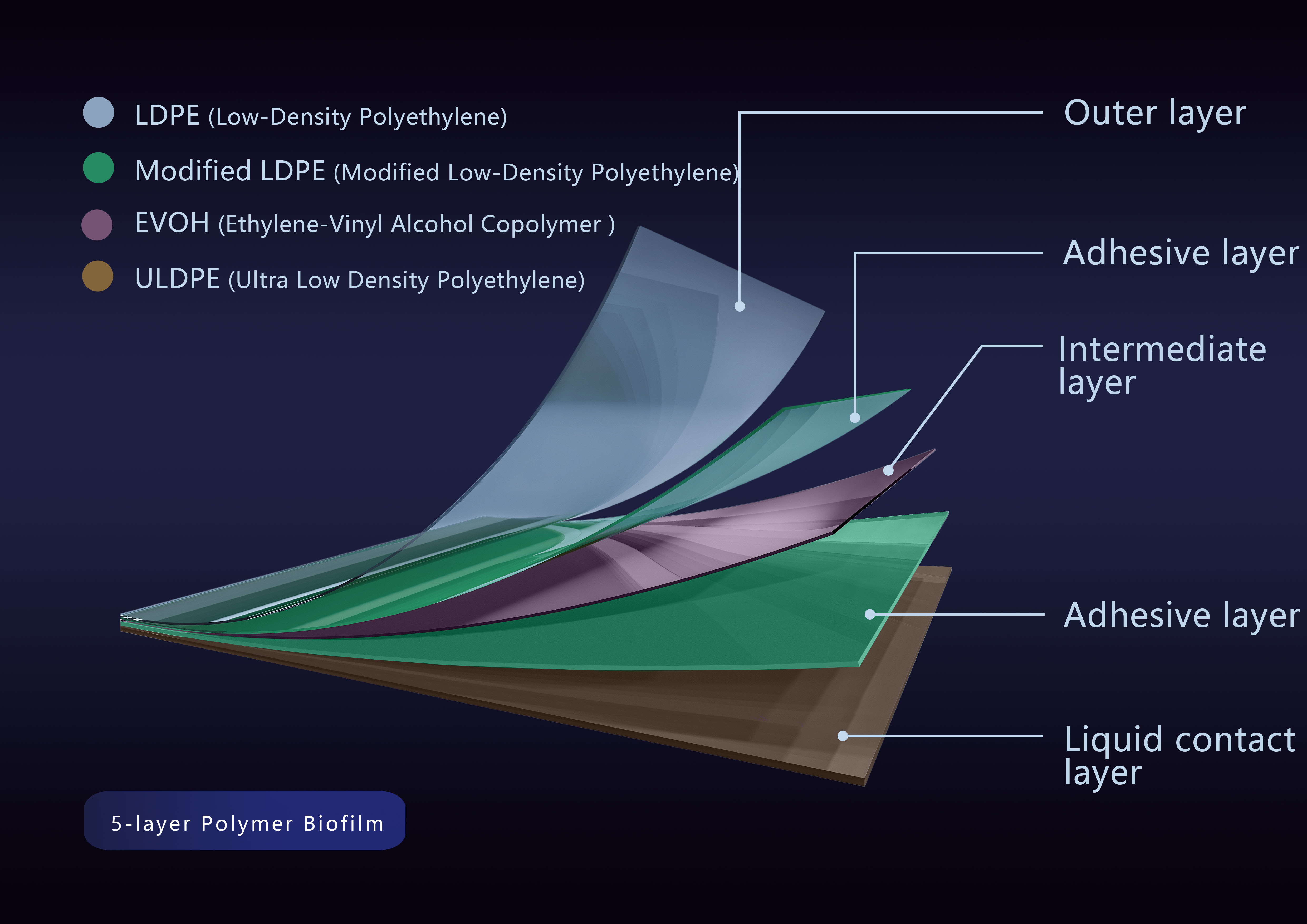
DuoFilm?-003 multilayer structure diagram
DuoFilm?-003 is mainly used for single-use cell culture bags, including single-use Wave-type bioreactors and single-use stirred tank bioreactors, and can provide volume specifications up to 2,000 L. This series of products has been extensively tested for cell culture and can support the culture requirements of different cell lines to obtain satisfactory cell culture results and product quality.
In addition, Duoning Biotech provides fully pre-assembled, pre-sterilized and customized single-use tube sets for multiple key unit operations in the biopharmaceutical production process, including single-use chromatography, ultrafiltration, virus filtration, and fill/finish. Such tube sets can be designed using parts manufactured internally or from qualified suppliers certified by customers and adopt an open architecture design to meet the operating requirements of different existing hardware equipment, which can fully meet the process requirements. Single-use tube sets for key unit operations including chromatography and ultrafiltration have been extensively tested by a large number of users.
Copyright ? Shanghai Duoning Biotechnology Co., Ltd. All Rights Reserved Sitemap | Technical Support: 
Message-